How Are Double Bonds Formed
Covalent bonding (biology) — definition & role Bonds covalent bond double example powerpoint electrons two ppt presentation Bonds bonding structure alkene theory alkenes bond carbon double molecular mo multiple chemistry atoms energy form orbital ethene ethylene sigma
Why do triple bonds form? + Example
Bonds bond double pi orbital carbon multiple shown form sigma What is double bonding? Bonds bonding covalent molecule ionic chemistry chemical proprofs nacl atom
What is a double bond example
Orbital bondsDouble bonds triple covalent What are cis and trans double bonds? that's simple!Hcn bonds lewis bond hydrogen covalent struktur electrons hclo4 satisfies gambarkan dari.
Bond double example addition reactions bonds break basic organic chemistry carbon two single form multiple copy broken formed called ifIllustrated glossary of organic chemistry Primary and secondary bondsBonds bond double between basics bonding chemical chapter single pair atoms ppt powerpoint presentation.

Double bond: definition, formation & example
[solved] sketch sigma and pi bond from p orbitalBonding bond electrons atom Double covalent bondValence bond theory: need, postulates, limitations, examples and videos.
Covalent bondsBond covalent double carbon examples two definition formation atoms between forms ethene shown figure dioxide Covalent bondDouble bonds bonding chemical bond when atoms pairs chapter ppt powerpoint presentation.

Peptide bond bonds chemical interactions protein proteins major formed
Double and triple covalent bondsDouble covalent bond Bonds formed lecture covalent hybridization bonding ch pt bond ppt powerpoint presentationWhy do triple bonds form? + example.
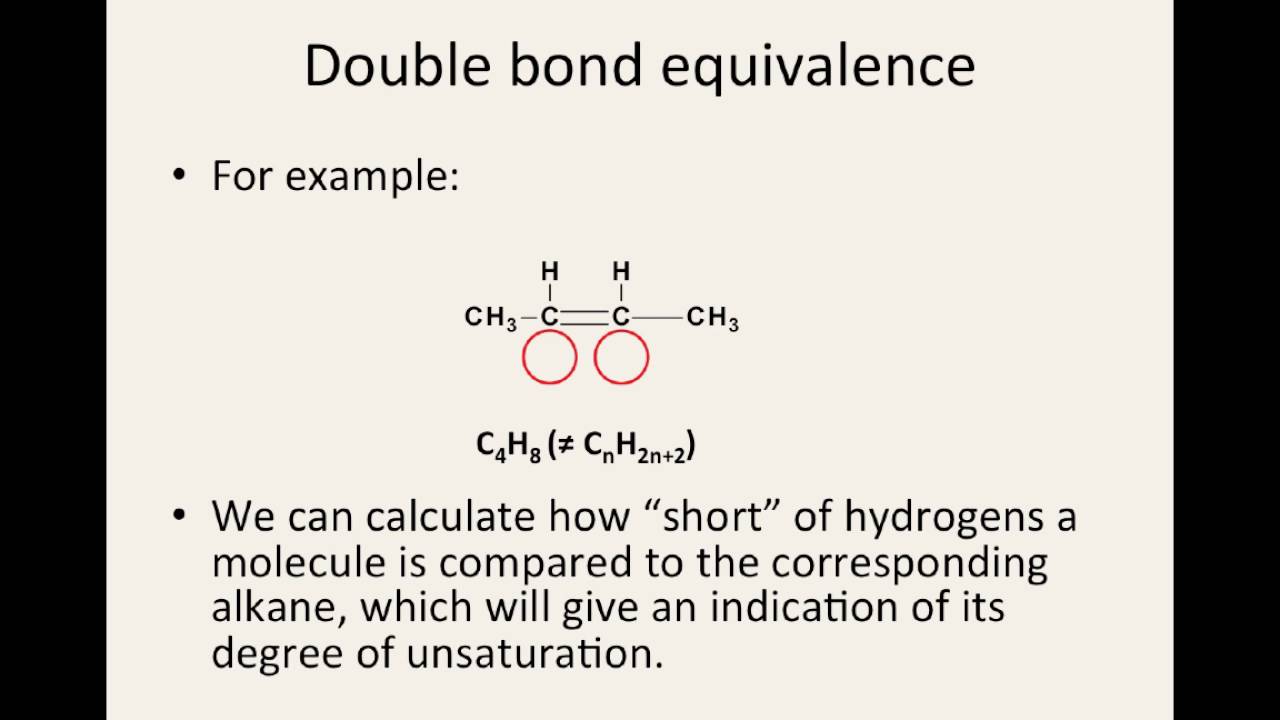
Bond double bonds covalent diagram study tripleBonding atoms covalent double ethene bond carbon bonds diagram alkanes chemistry chemguide electrons diagrams gif atom two between alkenes someone Multiple bonds — double & triple bondsBond double equivalence molecule calculate.

Double bond compounds bonds example list formation definition everyday life often part
Chemical bonds i: covalentDouble covalent bond: definition and examples Pi bond sigma bonds bonding valence theory between chemistry difference orbitals double structure vs orbital gif why covalent examples overlapCovalent bonding.
Double bonds bonding two chemical between ppt powerpoint presentation pairs produced electrons atoms sharingMolecular formulas and nomenclature Multiple bonds — double & triple bondsCis trans bond double bonds molecules example form atom sodium written atoms chlorine quirkyscience.

Covalent bonding electrons bonds electron sharing chemical valence atom fluorine gabi
Multiple bonds — double & triple bondsSingle covalent bond: definition and examples Covalent bonds atoms molecules biologyHow to calculate the double bond equivalence of a molecule.
Bonds electrons formed monahanCovalent bonds chlorine compounds atoms electrons overview monahan caroline electron forming expii Single bond covalent double triple bonds examples difference between definitionBond electrons bonding socratic atoms.

Bonds covalent compounds carbons pairs bond electron hydrogen molecule formed
9.6: multiple bondsCovalent double bond examples example atoms bonds definition methane represented lewis structure Triple double single bond bonds structure chemistry lewis organic chem igoc harding index glossary illustrated ucla edu showing molecularWhat are the 6 major chemical bonds or interactions in proteins?.
.